Crystallization of 4'-Hydroxyacetophenone from Water: Control of Polymorphism via Phase Diagram Studies
Cryst. Growth Des. 2012, 12, 2932−2941. DOI: 10.1021/cg300134z
Abstract
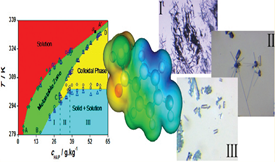
The preparation of polymorphs and solvates and the characterization of their stability domains have received considerable attention in recent years, due to the importance of these studies for fundamental research and for the production of new materials for task-specific applications. In this work, the selective and reproducible crystallization of different solid forms of 4′-hydroxyacetophenone (HAP) from water was investigated, through the determination of a temperature–concentration (T–cHAP) phase diagram. This determination was mainly based on gravimetric solubility measurements, slurry tests, and metastable zone width (MZW) studies with thermometric and turbidity detection. The experimental conditions for the formation of five different HAP phases by cooling crystallization could be established: the previously characterized anhydrous forms I and II and the hydrate HAP·1.5H2O (H1), and two new hydrates, one of stoichiometry HAP·3H2O (H2) and another (H3) which proved too unstable for a stoichiometry determination. The crystallization precedence of the various phases, their approximate lifetimes, and transformation sequences could also be elucidated. It was finally found that for a specific T–cHAP domain the crystallization of HAP solid phases was mediated by a colloidal dispersion. Preliminary dynamic light scattering experiments indicated that this dispersion consisted of particles with diameters in the range of 100–800 nm.
Return Previous Next