Polymorphism in 4-Hydroxybenzaldehyde: A Crystal Packing and Thermodynamic Study
Abstract
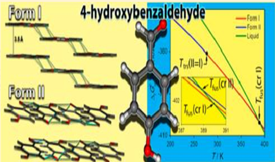
A procedure for the selective and reproducible preparation of the two known 4-hydroxybezaldehyde polymorphs was developed, based on the investigation of their relative stabilities by differential scanning calorimetry and solubility studies. From the obtained results, the stability domains of the two forms could be quantitatively represented in a ΔfGom −T phase diagram. The system was found to be enantiotropic: form II is more stable than form I up to 277±1 K; above this temperature, the stability order is reversed, and the fusion of form I subsequently occurs at 389.9 ± 0.2 K. Analysis of the crystal structures revealed that in both polymorphs the 4-hydroxybezaldehyde molecule exhibits the OH and C(O)H substituents in a Z conformation, which, according to B3LYP/6-31G(d,p) calculations, is more stable than the E conformation by only 0.4 kJ·mol −1. The two forms are monoclinic, space group P21/c, Z'/Z = 1/4, and have essentially identical densities at ambient temperature (1.358 g/cm for form I; 1.357 g/cm for form II), but differ in their packing. These differences are discussed, and the dissimilarities in the interactions sustaining the packing are highlighted using Hirshfeld surfaces. Finally, the relative stability and volumetric properties of both forms are analyzed by molecular dynamics simulations.
Return Previous Next