From Molecules to Crystals: The Solvent Plays an Active Role Throughout the Nucleation Pathway of Molecular Organic Crystals
C.E.S. Bernardes, M.L.S.M. Lopes, J.R. Ascenso, M.E. Minas da Piedade
Cryst. Growth Des. 2014, 14, 5436−5441. DOI: 10.1021/cg500609g
Abstract
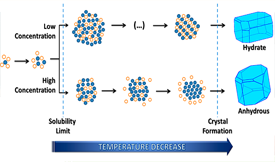
Crystallization is indisputably one of the oldest and most widely used purification methods. Despite this fact, our current understanding of the early stages of crystallization is still in its infancy. In this work dynamic light scattering and proton nuclear magnetic resonance were used to investigate the changes occurring in 4'-hydroxyacetophenone colloidal particles, as they form in a supersaturated aqueous solution and evolve toward anhydrous or hydrate materials during a cooling crystallization process. In the concentration range probed, the particles are initially composed by both solute and water. If the outcome of crystallization is an anhydrous phase, a complete loss of solvent from the particles is progressively observed up to the onset of crystal precipitation. These findings provide unique experimental evidence that the role of solvent in the formation of crystals can go well beyond influencing the self-assembly and clustering of solute molecules prior to nucleation.
Return Previous Next