The Standard Molar Enthalpy of the Base Catalysed Hydrolysis of Methylparaben Revisited
R.N. Bento, M.A. Rendas, V.A.R. Semedo, C.E.S. Bernardes, M.S.C.S. Santos, H.P. Diogo, F. Antunes, M.E. Minas da Piedade
Abstract
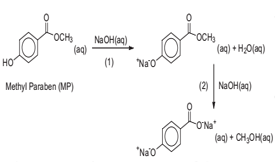
The standard molar enthalpy of the base catalysed hydrolysis of methyl paraben, DrHo m, was obtained at 298.02 K, 303.04 K and 310.01 K. The determination relied on isothermal microcalorimetry measurements and on a thermochemical scheme that, in contrast with previous strategies, does not require kinetic information. The results obtained were ΔrHmo = -(50.99 ± 0.61) kJ·mol-1 at 298.02 K, ΔrHmo = -(50.60 ± 0.82) kJ·mol-1 at 303.04 K, and ΔrHmo = -(49.64 ± 0.90) kJ·mol-1 at 310.01 K. The ΔrHmo value at 298 K is in very good agreement with the previously recommended benchmark at this temperature. The current procedure allowed, however, a 7-fold improvement in precision. It was also found that the ΔrHmo values show a linear variation with temperature. The fact that this variation is small lends support to the previous assumption of a negligible temperature dependency of ΔrHmo around 298 K. The present work resolves some important discrepancies in the reported standard molar enthalpies of the base catalysed hydrolysis of methyl paraben. Such inconsistencies can have a considerable impact on the determination of the effective volume of flow-through calorimetric apparatus. This parameter is essential to obtain kinetic and thermodynamic information on the growth, metabolism, and adaptation of living cells from flow calorimetry experiments. This paper reports an experimental and theoretical study on the structural and energetic characterization of the 2-mercaptoimidazole (2-MI) in the solid and in the gaseous phases. The single crystal X-ray diffraction determinations on the anhydrous and hemihydrate 2-MI forms were carried out at T = (296 ± 2) K and T = (150 ± 2) K, respectively, and suggest that in both forms the 2-MI molecule is closer to the thione conformation, albeit some single bond character is possible. The energy of combustion of the title compound was measured by rotating-bomb combustion calorimetry, being used to derive the corresponding enthalpy of formation in the crystalline-phase. The enthalpy of sublimation of 2-MI, at T = 298.15 K, was obtained from high temperature Calvet microcalorimetry measurements. These two parameters yielded the gas-phase enthalpy of formation, allowing the inherent energetic analysis of the molecule. This result was discussed together with the corresponding predictions for 2-MI and its tautomer, 1,3-dihydro-2H-imidazole-2-thione, by the G3 method. The dehydration reaction of 2-MI·0.5H2O(cr) was also investigated and the corresponding enthalpy of dehydration was determined by Calvet microcalorimetry.
Return Previous Next