A New Polymorph of 4'-Hydroxyvalerophenone Revealed by Thermoanalytical and X-ray Diffraction Studies
C.S.D. Lopes, C.E.S. Bernardes, M.F.M. Piedade, H.P. Diogo, M.E. Minas da Piedade
Eur. Phys. J. 2017 226, 849-855.
Abstract
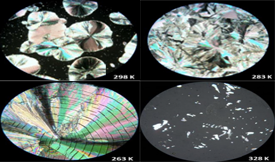
A new polymorph of 1-(4-hydroxyphenyl)pentan-1-one (4'-hydroxyvalerophenone, HVP) was identified by using differential scanning calorimetry, hot stage microscopy, and X-ray powder diffraction. This novel crystal form (form II) was obtained by crystallization from melt. It has a fusion temperature of Tfus = 324.3 ± 0.2 K and an enthalpy of fusion ∆fusHmo = 18.14 ± 0.18 kJ mol−1. These values are significantly lower than those observed for the previously known phase (form I, monoclinic, space group P21/c, Tfus = 335.6 ± 0.7 K; ∆fusHmo = 26.67 ± 0.04 kJ mol−1), which can be prepared by crystallization from ethanol. The results here obtained, therefore, suggest that form I is thermodynamically more stable than the newly identified form II and, furthermore, that the two polymorphs are monotropically related.
Return Previous Next