Structure–Property Relationships in Protic Ionic Liquids: a Thermochemical Study
J.E.S.J. Reid, F. Agapito, C.E.S. Bernardes, F. Martins, A.J. Walker, S. Shimizu, M.E. Minas da Piedade
Phys. Chem. Chem. Phys. 2017, 19, 19928-19936.
Abstract
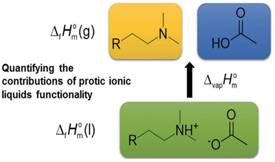
How does cation functionality influence the strength of intermolecular interactions in protic ionic liquids (PILs)? Quantifying the energetics of PILs can be an invaluable tool to answer this fundamental question. With this in view, we have determined the standard molar enthalpy of vaporization, ΔvapHom, and the standard molar enthalpy of formation, ΔfHom, of three tertiary ammonium acetate PILs with varying cation functionality, and of their corresponding precursor amines, through a combination of Calvet-drop microcalorimetry, solution calorimetry, and ab initio calculations. The obtained results suggest that these PILs vaporize as their neutral acid and base precursors. We also found a strong correlation between ΔvapHom of the PILs and of their corresponding amines. This suggests that, within this series of PILs, the influence of cation modification on their cohesive energies follows a group additivity rule. Finally, no correlation between the ΔvapHom of PILs and the extent of proton transfer, as estimated from the difference in aqueous pKa between the precursor acid and the conjugate acid of the precursor base, was observed.
Return Previous Next