Modeling Halogen Bonds in Ionic Liquids: A Force Field for Imidazolium and Halo-Imidazolium Derivatives
C.E.S. Bernardes, J.N. Canongia Lopes
J. Chem. Theory Comput. 2017,13, 6167-6176.
Abstract
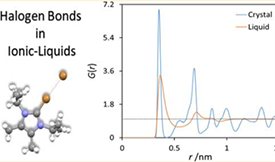
In this work, a force field for molecular dynamics and Monte Carlo simulations of ionic liquids containing imidazolium and halo-imidazolium derivatives is presented. This force field is an extension of the well-known CL&P and OPLS-AA models and was validated by comparing predicted crystalline structures for 22 ionic liquid compounds with the corresponding data deposited at the Cambridge Structural Database. The obtained results indicate that the proposed force field extension allows the reproduction of the crystal data with an absolute average deviation lower than 2.4%. Finally, it was also established that the halogen atoms covalently bound to the studied imidazolium cations are positively charged and do not exhibit a so-called σ-hole feature. For this reason, the formation of halogen bonds in the proposed force field appears naturally from the parametrized atomic pointcharge distribution, without the necessity of any extra interaction sites
Return Previous Next