A New Thermodynamically Favored Flubendazole/Maleic Acid Binary Crystal Form: Structure, Energetics, and in Silico PBPK Model-Based Investigation
G.L.B. Araujo, F.F. Ferreira, C.E.S. Bernardes, J.A.P. Sato, et. al.
Cryst. Growth Des. 2018, 15, 5349-5360.
Abstract
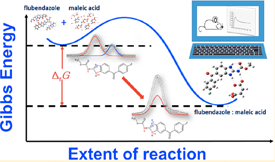
The use of flubendazole (FBZ) in the treatment of lymphatic filariasis and onchocerciasis (two high incidence neglected tropical diseases) has been hampered by its poor aqueous solubility. A material consisting of binary flubendazole/maleic acid crystals (FBZ/MA), showing considerably improved solubility and dissolution rate relative to flubendazole alone, has been prepared in this work through solvent assisted mechanical grinding. The identification of FBZ/MA as a binary crystalline compound with salt character (proton transfer from MA to FBZ) relied on the combined results of powder X-ray diffraction, Raman spectroscopy, attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), thermogravimetry (TG), and differential scanning calorimetry (DSC). Isothermal solution microcalorimetry studies further suggested that the direct formation of FBZ/MA from its precursors in the solid state is thermodynamically favored. A comparison of the in silico pharmacokinetic performance of the FBZ/MA with that of pure FBZ based on a rat fasted physiology model indicated that the absorption rate, mean plasma peak concentration, and absorption extension of FBZ/MA were ∼2.6 times, ∼1.4 times, and 60% larger, respectively, than those of FBZ. The results here obtained therefore suggest that the new FBZ/MA salt has a considerable potential for the development of stable and affordable pharmaceutical formulations with improved dissolution and pharmacokinetic properties. Finally, powder X-ray diffraction studies also led to the first determination of the crystal structure of FBZ.
Return Previous Next