Energetics of Dehydroepiandrosterone Polymorphs I and II from Solution and Drop-Sublimation Calvet Microcalorimetry Measurements
C.E.S. Bernardes, I.O. Feliciano, C. Naese, F. Emmerling, M.E. Minas da Piedade
J. Chem. Thermodyn. 2023, 186, 107137.
DOI: 10.1016/j.jct.2023.107137.
Abstract
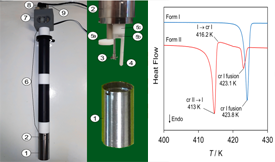
The lattice enthalpies and monotropic relationship of two dehydroepiandrosterone (DEHA) polymorphs (forms I and II) were evaluated through a combination of differential scanning calorimetry (DSC), isothermal solution microcalorimetry, and drop-sublimation Calvet microcalorimetry experiments. The standard molar enthalpy of transition between both forms was determined as ΔtrsHmo(II → I, 298.15 K) = −0.90 ± 0.07 kJ mol-1 and ΔtrsHmo(II → I, 417.8 K) = − 1.7 ± 1.0 kJ mol-1, from measurements of standard molar enthalpies of solution in dimethyl sulfoxide and enthalpies of fusion, respectively. Drop-sublimation Calvet microcalorimetry experiments on form I led to ΔsubHmo(cr I, 298.15 K) = 132.0±3.3 kJ mol-1. This result, when combined with the more precise ΔtrsHmo(II → I) value obtained by solution calorimetry, afforded ΔsubHmo(cr II, 298.15 K) = 131.1±3.3 kJ mol-1. The overall data indicate that on enthalpic grounds form I is more stable than form II from 298.15 K up to fusion. This conclusion, and the fact that DSC experiments indicated that form I has also a considerably higher temperature fusion, namely, Tfus(cr I)= 422.5±0.2 K and Tfus(cr II) = 413.1±0.2 K, suggest that the two polymorphs are monotropically related.
Return Previous Next